Abstract
Background: Patients with AML harboring FLT3 mutations have poor clinical outcomes. Furthermore, FLT3 mutations frequently co-occur with other driver mutations, such as NPM1 with DNMT3A, WT1, and RUNX1, that are associated with poor prognosis. Crenolanib is a highly potent and specific type-I FLT3 inhibitor, which has shown promising safety and efficacy in combination with chemotherapy. Here we report the outcomes of newly diagnosed FLT3 mutated AML patients treated with crenolanib and intensive 7 + 3 based chemotherapy (NCT02283177) by baseline genomic profile.
Methods: Patients were treated on clinical trial with 7+3 induction chemotherapy combined with crenolanib, consolidation with high-dose cytarabine (HiDAC) combined with crenolanib, and/or allo-HCT followed by crenolanib maintenance. Of 44 pts enrolled and treated, 36 had sequencing performed at baseline. The median survival follow-up for these patients was 20.7 months with data cut off July 25, 2018. A post hoc analysis was performed to assess the impact of genomic profile on patient outcomes using published data from the German-Austrian AML Study Group as a historical control.
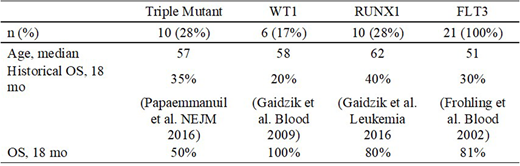
Results: Concurrent FLT3-ITD, NPM1, and DNMT3A mutations ("triple mutant") were present in 10 pts. These patients demonstrated improved OS with crenolanib treatment compared with historical controls. Similarly, patients with FLT3-ITD and WT1 mutations (n = 6) showed dramatically improved outcomes, with no deaths occurring by 18 months. Patients with FLT3 (ITD or TKD) and RUNX1 mutations (n = 10) also had improved OS.
Conclusions: This analysis suggests that adding a potent pan-FLT3 inhibitor can overcome the poor prognostic implication of adverse mutations co-occurring with mutated FLT3. These data support the combination of crenolanib with chemotherapy to improve the overall outcome of FLT3 mutated AML with diverse mutational profiles. Hence, a randomized trial has been initiated of standard chemotherapy combined with either crenolanib or midostaurin in newly diagnosed patients with FLT3-mutant AML (NCT03258931).
Goldberg:AROG: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; Abbvie: Research Funding. Collins:Arog Pharmaceuticals: Research Funding; Celgene Corporation: Research Funding; Bristol Myers Squibb: Research Funding; Agios: Research Funding. Stone:Ono: Consultancy; Novartis: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Argenx: Other: Data and Safety Monitoring Board; Arog: Consultancy, Research Funding; Merck: Consultancy; Fujifilm: Consultancy; Jazz: Consultancy; Orsenix: Consultancy; Astellas: Consultancy; Celgene: Consultancy, Other: Data and Safety Monitoring Board, Steering Committee; AbbVie: Consultancy; Amgen: Consultancy; Otsuka: Consultancy; Pfizer: Consultancy; Sumitomo: Consultancy; Cornerstone: Consultancy. Walter:Actinium Pharmaceuticals, Inc: Other: Clinical Trial support , Research Funding; Amgen Inc: Other: Clinical Trial Support, Research Funding; Amphivena Therapeutics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding; Aptevo Therapeutics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding; Covagen AG: Consultancy, Other: Clinical Trial Support, Research Funding; Boehringer Ingelheim Pharma GmbH & Co. KG: Consultancy; Pfizer, Inc: Consultancy; Seattle Genetics, Inc: Consultancy, Other: Clinical Trial Support, Research Funding. Wang:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Jazz: Speakers Bureau; Jazz: Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Novartis: Speakers Bureau. Tallman:AROG: Research Funding; AbbVie: Research Funding; Orsenix: Other: Advisory board; Daiichi-Sankyo: Other: Advisory board; ADC Therapeutics: Research Funding; Cellerant: Research Funding; BioSight: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal